1. Species information
- Scientific name: Pseudolestes mirabilis
- General name: Phoenix
- Classification: Insecta -> Odonata -> Pseudolestidae -> Zygoptera -> Pseudolestes
- Habitat: Adults can be found in most areas in Hainan, in the small to moderate-sized stream in well-forested uplands; and also sometimes encountered in more open habitat; Larvae were found in both shady and open montane streams with stony substrates. Some even occurred in very small “puddles” covered with dense vegetation formed by discontinuous streams.
- Distribution: Hainan, China (endemic)
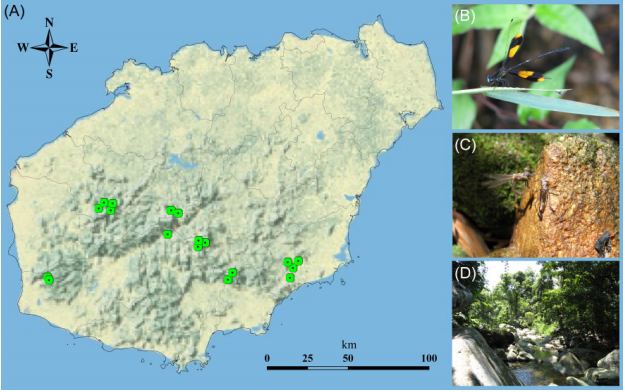
Figure 1. (A) Map of the occurrence records of P. mirabilis in Hainan Island. (B) Adult, (C) larva and (D) habitat. (Zhu et al., 2015; permitted to use)
- Description: the adult is fairly small. Males have a blue face, black-brown eyes, and black head. The thorax is matt black. The forewing is long and narrow; the hind wings are much shorter and broader. The hind wings are black on the dorsal surface, with an extensive metallic gold. The hind wings underside is black, too, marked extensively with white from the nodus to the tip. Females lack the blue face and have more extensive yellow streaking on the thorax. The hindwing is black on the distal half, tipped with white (Kirby, 1900; Reel & Zhang, 2015).

Figure 2. Adult of P. mirabilis (©Xin Yu, permitted to use)
- The larva is robust with a large head and small, relatively broad and robust thorax. Legs moderately short and strong, bearing scattered short spines and hairs, lacking bands or other marks. A tuft of abdominal gills is present ventrally on the final segment, the gills are paired and retractable. Cerci wholly concealed by expanded gill tufts in ventral view. Inflated sack-like caudal gills covered in short, stout setae; football-like in the final larva with blunt apical projections, slightly longer and finger-like in the earlier instar larva. Ground colour of body dark brown, lacking distinct bands or marks. (Yu & Bu, 2011; Yu & Bu, 2014).

Figure 3. (A) Dorsal and (B) ventral view of larvae of P. mirabilis (©Xin Yu, permitted to use)
Figure 4. (A) Alive larvae and (B) the young adult after eclosion (©Xin Yu, permitted to use)
After a boring and prim introduction of this species, let’s start the really interesting and exciting part!
What is P. mirabilis ?
P. mirabilis is remarkable as its beautiful morphology: blue face, black-gold hindwing and the beautiful ‘dance’. That’s the reason they are called as ‘Phoenix’. You can easily find this species in streams in Hainan Island, many literature and books said as ‘very widespread’ or ‘common’. However, the use of ‘common’ is a little bit misleading, because this kind of ‘common’ is limited in Hainan Island.
Longely species
Actually, P. mirabilis should be called as ‘very endemic’ or ‘very unique’. That is not only because of that this species is only distributed in Hainan Island, but because of its unique status in classification. For a long time, P. mirabilis is like a waif: it has no relative and has no idea about its own position in phylogeny. From 1900 to 2010, its classification position has changed many times. But finally, with the help of molecular data, the phylogenetic study suggested that it should go back to its own family (Dijkstra et al, 2014). What does it mean? It means that P. mirabilis finally finds its family, BUT this family only has one species, no other sisters or we have no idea about where its sisters are yet. It’s not normal in insects, which always has a lot of species in a family level group. Another significant character that helps it find its own family is its retractable gills. The Odonata abdominal gills only occur in larvae of Amphipterygidae, Euphaeidae and Polythoridae (Corbet, 1999; Sisby, 2001) and believed as a homologous organ which evolved independently (Yu & Bu, 2011; Yu & Bu, 2014).
It is a very special phenomenon in studies of Odonata that using overall larval characters to study about taxonomy and phylogeny. Some species like this one we are discussing, P. mirabilis, the characters of its adult may simply be the result of a rapid evolution and adaptation (Yu & Bu, 2011). Therefore, it is hard to place this kind of monotypic genus by analysis adult characters, even under a comprehensive molecular study (Bybee et al., 2008; Dijkstra et al., 2014; Wang et al., 2017). On the other hand, the larval characters may often be less affected by the radiation or adaptation than the adult. So the larvae of Odonata play important and unique roles in both taxonomy and phylogeny studies, which is very different to find in other insect groups.
Special ‘scales’ in hindwings
One more thing in morphology that makes P. mirabilis very unique is the special ‘scales’ in its hind wings. It’s a truth that the hind wings of many dragonflies could reflect beautiful colour. The reason why they could do it is that of the multilayer interference or caused by wing-membrane pigmentation (Prum et al., 2005; Schultz & Fincke, 2009; Nixon et al., 2017). P. mirabilis reflects brilliant white on the ventral side of its hindwings and a copper-gold colour on the dorsal side. Different with other Odonata wings, the whiteness on the wings of P. mirabilis results from light scattered by a specialized arrangement of flattened waxy fibres and the copper-gold colour is produced by pigment-based filtering of this light scatter (Nixon et al., 2017).
Beautiful ‘dancing’
The structure is the basis of behaviour. Since P. mirabilis has such special morphological characters, it may be related to its unique (if it has) behaviour. Actually, the answer is YES! There are more females than males in populations of P. mirabilis (Cordero–Rivera & Zhang, 2018), which means a war to compete for mates between males is inevitable. How damselfly fight? Two periods. The first period is flaunting, the exhibition of the coloured hindwings. The agonistic encounters between males were usually very short (less than 2 min) and consisted of a face–to–face display with both males maintaining a close distance while flying using only the forewings. It should be noted that NO other Odonata flies with only two wings in territorial contests. Furthermore, a small proportion of fights were escalated and lasted about one hour (Cordero–Rivera & Zhang, 2018). Let’s back to the structure of its hind wings, this structure provides a mechanism enabling P. mirabilis to display its bright wing colours efficiently for territorial signaling, both passively while perched, in which the sunlit copper-gold upperside is presented against a highly contrasting background of foliage, and actively in territorial contests in which the white underside is also presented (Nixon et al., 2017). Therefore, the intrasexual selection is behind the evolution of coloured wings in this species (Cordero–Rivera & Zhang, 2018).
The second period of competition is in population. Most of Odonata males will translocate sperm to their vesicle before each mating, but obviously, so far our P. mirabilis doesn’t want to be such normal. Males of P. mirabilis translocate sperm after copulation a behaviour that cannot be easily explained (Cordero–Rivera & Zhang, 2018).
‘Common’ or ‘rare’?
I really do not want to use ‘special’ to describe this species again since I have used too many times. I believe you could understand how important this species is. When people said it is a common or widespread species, does it mean this is safe under the crazy anthropic activities? Especially in Hainan Island, where there is a large number of estate development and tourism have swallowed quickly a large area of forests. In IUCN, the information of this species is DD (Data Deficient). Recently, couples of studies have done its distribution and population genetics (Zhu et al., 2015; Xue et al., 2017). The results indicated that P. mirabilis experienced significant genetic drift recently (last glaciation of Pleistocene), and since its population has a low genetic diversity. What it means? A high genetic diversity is a key to adapt or fight with the terrible environment, it allows your population carrying enough mutation to face to the environmental changes. Even there are several individuals that carrying the beneficial mutation, these mutations will disperse quickly in their population. That is the power of natural selection. That is also the reason why passenger pigeon extinct: whatever how large of your population or abundance, you cannot escape from this fast and strong selection which accompanied by a rapid loss of most mutations (Murray et al., 2017). Back to our P. mirabilis, the low genetic diversity also means that it has a low adaptive capacity to the environment, and could easily be suffered from surrounding disturbance (climate or human impact, whatever).
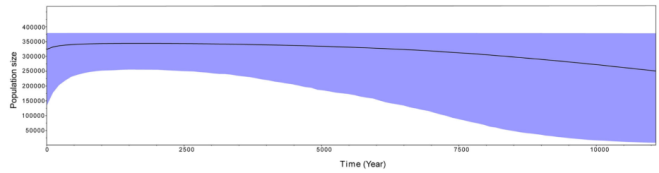
Figure 5. Historical demography inferred from mtDNA COI sequences in Bayesian skyline plots analysis. P. mirabilis experienced significant genetic drift recently. (Xue et al., 2017; permitted to use)
These studies also pointed out that some marginal small populations (such as several on the east coast) are already dying out. The relatively high population size and genetic diversity of the south-central population are under serious threat from tourism and urbanization, if not protection from rapid extinction.

Figure 6. (A) Estimations of gene flow for all populations implemented, arrows represent the direction, the green arrow means high gene flow. Circular dots indicate different populations and the different colour in dots represents different haplotypes. We could find that the population 6 in the eastern margin has a very low genetic diversity. (B) Predicted species distributions under current conditions. We could find that the centre of diversity is located in these regions that under serious threat from tourism and urbanization. (Xue et al., 2017; permitted to use)
References
- Bybee, S. M., Ogden, T. H., Branham, M. A., & Whiting, M. F. (2008). Molecules, morphology and fossils: a comprehensive approach to Odonate phylogeny and the evolution of the Odonate wing. Cladistics, 24(4), 477-514.
- Cordero–Rivera, A., & Zhang, H. (2018). Ethological uniqueness of a damselfly with no near relatives: the relevance of behaviour as part of biodiversity. Animal Biodiversity and Conservation, 41(1), 161-174.
- DijkstraI, K. D. B., Kalkman, V. J., Dow, R. A., Stokvis, F. R., & Van Tol, J. A. N. 2014. Redefining the damselfly families: a comprehensive molecular phylogeny of Zygoptera (Odonata). Systematic Entomology, 39(1), 68-96.
- Kirby, W. F. 1900. LXX.—On a small collection of Odonata (dragonflies) from Hainan, collected by the late John Whitehead. Journal of Natural History, 5(30), 530-539.
- Murray, G. G., Soares, A. E., Novak, B. J., Schaefer, N. K., Cahill, J. A., Baker, A. J., … & Gilbert, M. T. P. (2017). Natural selection shaped the rise and fall of passenger pigeon genomic diversity. Science, 358(6365), 951-954.
- Nixon, M. R., Orr, A. G., & Vukusic, P. (2017). Covert linear polarization signatures from brilliant white two-dimensional disordered wing structures of the phoenix damselfly. Journal of the Royal Society Interface, 14(130), 20170036.
- Prum, R. O., Cole, J. A., & Torres, R. H. (2004). Blue integumentary structural colours in dragonflies (Odonata) are not produced by incoherent Tyndall scattering. Journal of Experimental Biology, 207(22), 3999-4009.
- Reels, G. T., & Zhang, H. M. 2015. A field guide to the dragonflies of Hainan. Kadoorie Conservation China, Kadoorie Farm and Botanic Garden (eds). Joy of Nature–Hainan Wildlife Field Guide Series. Beijing.
- Schultz, T. D., & Fincke, O. M. (2009). Structural colours create a flashing cue for sexual recognition and male quality in a Neotropical giant damselfly. Functional Ecology, 23(4), 724-732.
- Wang, R., Yu, X., Xue, J., & Ning, X. (2017). Descriptions of larvae of Vestalaria venusta (Hämäläinen, 2004) and Matrona basilaris Selys, 1853 (Odonata: Calopterygidae). Zootaxa, 4306(4), 580-592.
- Xue, J., Yu, X., Zhang, H., Chen, X., & Bu, W. (2017). Population genetics and ecological niche modeling shed light on conservation of the island endemic damselfly Pseudolestes mirabilis (Odonata, Pseudolestidae). Hydrobiologia, 790(1), 273-286.
- Yu, X., & Bu, W. 2011. A description of the remarkable larva of Pseudolestes mirabilis Kirby (Odonata: Pseudolestidae). International Journal of Odonatology, 14(2), 105-110.
- Yu, X., & Bu, W. 2014. Notes on the retractabilty of gill tufts in Pseudolestes mirabilis (Zygoptera: Pseudolestidae). International Journal of Odonatology, 17(2-3), 123-126.
- Zhu, G., Yu, X., & Bu, W. 2015. Ecology and conservation of Pseudolestes mirabilis (Odonata: Zygoptera), a damselfly endemic to Hainan Island of China. Entomological Science, 18(1), 123-129.

 Notably large anterior middle eyes of M. magna with raised first pair of legs, imitating the antenna of ants
Notably large anterior middle eyes of M. magna with raised first pair of legs, imitating the antenna of ants


 Credit: LiCheng Shih
Credit: LiCheng Shih